Selecting Optimal Stainless Steels for Bio-Pharmaceutical Service
Technical Guide
Abstract
This paper describes the various grades of stainless steels and their characteristics which lead to applications in gas and fluid handling systems in process industries. Different melting techniques are discussed in terms of their influence on microstructural cleanness. These are related to fabrication and surface finish. A special quality of Type 316L, designated CarTech 316L-SCQ® Stainless suitable for the bio-pharmaceutical industry is introduced.
Introduction
Stainless steels have become important engineering materials for ultra clean gas supply equipment. Requirements include corrosion resistance, fabricability (machining and welding), electropolishing, and material purity. Improvements are attainable through new alloys, and via more sophisticated processing techniques applied to conventional alloys.
This presentation consists of two parts. The first is an introduction of a new level of quality available with CarTech 316L alloy. The focus is on cleanness levels available as a function of composition and melting practice, and the relationship to machinability , welding, and electro-polishing. The second part is a general overview of stainless steels, and details on the melting techniques available which can provide the improved cleanness.
Cleanness
Air melted CarTech 304L alloy and CarTech 316L alloy stainless steels have been successfully used for fittings, valves, tubing and other components of gas and fluid handling systems in many different industries. Occasional problems with "Ieakers" have demonstrated that the frequency and severity of randomly distributed inclusions are unsuitable for some critical applications. These deficiencies become most apparent on cross sections, under conditions of extremely high pressure or vacuum. Additionally, it has become apparent lately that the presence of discontinuities on internal surfaces resulting from the removal of inclusions which intersect the surface can trap or release contaminants which can affect end product purity and yields. For these reasons there is interest in measuring and controlling the inclusion content of the raw material, and justification for upgrading to premium melting techniques where cleanness levels can be guaranteed.
A commonly accepted practice for determining the inclusion content in steel is ASTM designation E45-87. In the microscopical methods inclusions are assigned to a category based on similarities in morphology and not necessarily on their chemical identity, Inclusion types are A (sulfides), B (alumina), C (silicates) and D (oxides) .Each field of the specimen is compared with the fields of a reference chart, and the inclusion rating for each type is recorded for both the thin and heavy series. Worst field ratings are calculated and used to establish conformance to specification limit.
Specimens for examination are usually selected at an intermediate billet size, and may be cut from locations representing the top and bottom of the first, middle and last ingot poured of a heat. The purpose is to qualify the heat before it is hot worked to the final rod size. During reduction by forging or rolling inclusions are elongated and broken up according to the degree of reduction of steel cross section. Broken stringered inclusions are classified as two distinct inclusions when they are separated by at least 0.005 inch of clear area.
Because the inclusion content of stainless steels melted in air can vary significantly and the solidification pattern for static cast ingots tends to segregate non-metallic compounds, JK (inclusion chart) limits are not normally quoted for air melted material. JK ratings around 3 are not unusual. By contrast, depending on whether single vacuum melting or double vacuum melting steps are employed, limits of 1 or less can be met. A comparison of the microstructure of air melt high residual sulfur CarTech 316L alloy and vacuum arc remelted low sulfur CarTech 316L alloy is shown in Figure 1.
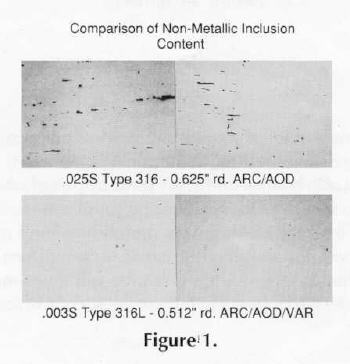
Fabrication
Residual levels of sulfur in austenitic stainless steels can influence machinability and weldability. In machining operations, sulfur acts as an internal chip breaker and offers some degree of lubricity to the cutting tool surfaces. But the improved rigidity of newer machine tool set-ups, and the easy ability to vary speeds and feeds afforded by CNC equipment has enhanced the capability to machine heats with sulfur controlled to very, very low levels. Usually the lower the sulfur level the greater the tendency to burnish the surface which improves surface luster.
With respect to weldability, it is known that sulfur influences weld penetration and bead contour. Penetration ratio (ratio of depth to bead width) increases with an increase in sulfur content up to about 0.03%. The weld bead geometry (weld meandering) can be a problem if components with significantly different sulfur levels are joined. Good welds are possible between two compositions if the difference in sulfur level is less than about 0.010%. Also, slight adjustments in weld parameters make possible full penetration welds with S as low as 0.001%.
Passivation / ElectroPolishing
Sulfides and other non-metallic inclusions intersect planes which are not exactly parallel with the rolling direction, i.e. cross sections and internally generated surfaces in parts made from bar stock and tubing. Since these are non-metallics and lack corrosion resistance, they are removed during passivation and electropolishing operations. Passivation is a treatment in nitric acid designed to remove any contamination by iron particles from fabrication operations. The nitric acid does not attack the stainless matrix, but it does remove non-metallic inclusions, leaving microscopic voids. Electropolishing actually removes a small amount of metal from the surface, in addition to aggressively removing non-metallics. Clearly, the fewer intersections of non-metallics with the surface, the less the number of sites for process contaminants to lodge. Even slight corrosion of the metallic components in service can expose fresh numbers of non-metallics, so the frequency and severity of such inclusions is an important consideration.
Material Selection / Availability
Process engineers responsible for designing and building equipment to handle fluid and gas flow can be expected to respond to the prospect of "new, improved, better". The desirability of improved cleanness has been apparent after technical analysis of numerous plant problems. But it is not always possible to have what you want when you want it. A minimum heat size of a specific analysis is normally in excess of 10,000 pounds, and the minimum quantity required to set up a modern rolling mill to roll a specific size is in the range of 2,000 pounds. What has long been necessary is sufficient demand for a variety of products in a wide range of applications to establish the material as a viable standard.
The semi-conductor industry is relentlessly pursuing improvements to internal surfaces of gas delivery systems for chip processing. The same driving force for minimizing contamination and increasing product yield is present in the bio-pharmaceutical industry, and the fiber-optics industry. Users of vacuum systems and high pressure systems can benefit from the synergistic upgrading from CarTech 304L alloy to CarTech 316L alloy and further to the use of a remelted product.
One such product line currently available is Carpenter Technology Corporation's CarTech 316L-SCQ Stainless. This description is an umbrella for several different analyses and melting practice combinations, to provide the most cost-effective starting material for different end uses. The program includes in-process billet inventory to decrease mill lead times and reduce the minimum order quantity. Included in the product line is a low residual sulfur analysis which is electro-slag (ESR) remelted for heavy wall containers such as gas cylinders, an intermediate sulfur analysis (0.005-0.015%) which is air melt plus vacuum arc remelt (AOD + VAR) and a very low residual sulfur analysis which is double vacuum melted (VIM + VAR). Product forms which can be made include extrusion and forging billet and bar, machining bar, wire, and strip.
Other Varieties of Stainless
Everyone knows that iron and plain carbon steels will uniformly corrode (rust) when exposed to a moist atmosphere or mild corrodent. Seventy-five years ago it was discovered that a minimum of about 12% chromium (Cr) would provide both oxidation and corrosion resistance to iron base alloys. This has become a suitable definition for stainless steel. The "passivity" of such alloys is due to an impervious chromium oxide film on the surface, which is formed and maintained under chemically oxidizing or neutral conditions.
Other elements affect the physical and mechanical properties, and corrosion resistance, by their influence on the arrangement of atoms within the crystal structure. The microstructures produced provide a means for classifying the various alloys and generalizing about their attributes. Among the most important elements is carbon (C) which influences whether the alloy can be thermally hardened or not, and correlates with the strength and toughness achieved. Carbon can readily form a stable compound with chromium (chromium carbide}, and if chromium is thereby prevented from contributing to the oxide film, serious consequences for corrosion resistance can result. Nickel (Ni), in levels exceeding about 8 percent, changes the physical and mechanical properties dramatically, and extends the range of corrosive environments in which these alloys are used. Manganese (Mn) and copper (Cu} perform some of the same functions as nickel. Molybdenum (Mo) can provide a significant improvement in resistance to pitting and crevice corrosion, depending on the level used. Sulfur (S) and selenium (Se) will improve machinability, while columbium (Cb) and titanium (Ti) provide one means for allowing components to be used in the as-welded condition. There are other residual elements, not intentionally added but present from the charge materials that mayor may not have metallurgical importance.
Discussion of the different types of stainless alloys, especially from a technical standpoint is beyond the scope of this paper. A comprehensive reference is the Handbook of Stainless Steels by Peckner and Bernstein1, and an extensive list of topics is covered in various volumes of the Metals Handbook by ASM2. The commonly recognized categories and a brief summary of characteristics is shown in Figure 2.
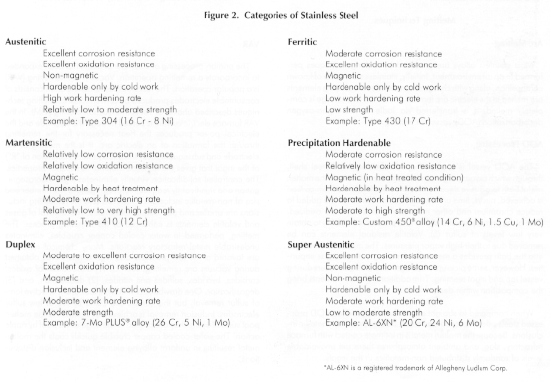
For applications in the chemical process, food handling, and pharmaceutical industries, CarTech 304 is generally considered to be the alloy with the minimum level of corrosion resistance suitable for general usage. Because of the slightly higher nickel level, and the intentional addition of approximately 2 percent molybdenum, CarTech 316 is usually specified. This provides improved pitting and crevice corrosion resistance in the presence of chlorides, which are particularly troublesome with stainless steels whenever moisture is present. CarTech 316L alloy, where the L denotes low carbon content (less than 0.03 percent) precludes the formation of a continuous network of chromium carbides in grain boundaries should the alloy be exposed to temperatures between 800° and 1500°F. This permits the use of components in the as-welded condition without risk of intergranular corrosion.
CarTech 316 and CarTech 316L alloy have been extensively employed for components manufactured from billet, bar, wire, sheet, plate and tubing. Where problems have arisen due to high chloride concentrations, additional chromium, molybdenum and nitrogen, up to the limit where deleterious second phases may form in the microstructure, have been used to provide better corrosion resistance at reasonable cost. Examples of suitable alloys are 22Cr-13Ni-5Mn and AL6XN. For the most aggressive environments, it may be necessary to go all the way to nickel base alloys with high levels of chromium and molybdenum.
Melting Techniques
Arc Melting
Most stainless alloys are produced by melting techniques performed in an air environment. Initially, stainless steel scrap of known composition, along with charge chrome and some alloying elements are melted in the electric arc furnace. When the total charge is completely melted, it is transferred via ladle to the argon-oxygen decarburization (AOD) vessel for refining.
AOD Processing
The AOD vessel is a pear shaped refractory lined steel shell, through which oxygen, argon and nitrogen are blown into the molten metal bath to achieve decarburization. After the desired aim carbon is achieved, alloys, lime and ferro-silicon or aluminum are added to reduce chromium and other metallic elements that were oxidized. One of the primary benefits of the AOD process is the ability to obtain very low levels of sulfur (S). Volatile residual elements can be removed due to their high vapor pressures. The injection of inert gas into the bath provides a means for removing unwanted gas impurities. However, some pick-up in hydrogen and nitrogen occurs during vessel tap and ingot teeming. Trim additions of alloy elements bring the composition within desired limits.
When compared to straight electric furnace product, AOD processed steels offer improved cleanness, due to lower non-metallic inclusions. Because the molten metal is in intimate contact with furnace refractory, slag, and ambient atmospheres there are unavoidable levels of randomly distributed non-metallics in the ingots.
VAR
The primary pracessing af a number af alloys has been expanded to incorporate a remelting operation. Vacuum Arc Remelting (VAR) is a popular operation. The feedstock for remelting often consists of consumable electrodes produced by conventional air melting techniques described above, or vacuum induction melting (VIM). In the VAR furnace electric current is passed through the electrode and the electrical power produces the heat necessary for the remelting through the formation of an electric arc. It is the melting of the electrode and subsequent resolidification (another definition of "R") of the ingot that gives the remelted material its superior properties. The controlled solidification virtually eliminates ingot macrosegregation and significantly reduces microsegregation. The number and size of non-metallic inclusions is reduced and the remaining inclusions are smaller and distributed more evenly. The removal of gases and volatile elements is enhanced by the remelting process. The melting, performed in water cooled copper crucibles, eliminates undesirable metal/refractory reactions. Many chemical reactions are favored at the low pressures and high temperatures obtained during vacuum arc remelting, such as (1) dissociation of oxides,carbides, hydrides, sulfides and nitrides, (2) deoxidation, and (3) degasification. One drawback of VAR is the lack of any effective form of sulfur removal, but this can be overcome by use of low sulfur electrodes. Inclusion removal is achieved by flotation to the molten pool surface where they are pushed to the edge of the pool by ripple action. The water-cooled copper crucible quickly cools the molten metal resulting in uniform alloying element and inclusion distributions.
ESR
The electroslag remelting process (ESR) is similar in many respects to VAR. The process combines fully controlled melting conditions with fully controlled freezing conditions, but remelting occurs through a chemically active slag as opposed to melting under a vacuum.
Single phase AC power is applied to the electrode, which is aligned above the water-cooled mold. The electrode is immersed in the slag, and droplets of molten metal form on the bottom of the electrode and fall through the slag to the metal pool. The slag is the heating component of the system, it acts as a solvent for non-metallics and protects the molten metal from contamination, and acts as a mold lining. The water-cooled mold promotes a shallow molten pool which reduces microsegregation, increases chemical homogeneity, improves inclusion distribution and increases ingot soundness.
VIM
The electrode which is remelted in the VAR or ESR process can also be created via vacuum induction melting (VIM). Two major benefits are reduced gas contentand good chemistry control. Disadvantages include contact of the molten metal with the furnace refractory, and inability to use reactive slags.
The vacuum induction process uses an airtight vessel or vacuum chamber which completely encloses the melting equipment. Mechanical vacuum systems, in conjunction with an oil diffusion system, are used to evacuate the chamber. A water cooled copper coil surrounds the furnace and heating is accomplished by sending an electric current through the coil. This current sets up a magnetic field that interacts with the charge material, creating frictional heat between the atoms. The charge materials must be of the composition desired -only relatively small quantities of late alloy additions can be made via a recharge system. When the metal is molten the furnace is tilted 90 degrees enabling the heat to be poured over a spout and into an awaiting mold. A mold turntable is used to position empty molds under the pouring spout.
Conclusion
Raw material is but the first step to achieving a high purity distribution system that doesn't contaminate the process. Concurrent improvements in machining techniques, electropolishing, surface characteristic measurement, and joining processes are equally important. Process control is essential as well. Taken together, these factors assure that the quality is indeed designed in.
The availability of CarTech 316L-SCQ Stainless provides, for the first time, economical quantities of an improved quality level that can contribute to less contamination and improved product yields. Industry-wide and company proprietary specifications have been written to define the quality assurance requirements. Cooperation between end users and material suppliers has achieved a major improvement in reliability of finished components.
References:
1. Handbook of Stainless Steels. Donald Peckner and I. M. Bernstein. McCraw Hill Book Co., N.Y., 1977
2. Metals Handbook, Ninth Edition, published by ASM International, Metals Park, OH 44073
***
By Norman B. Schmidt
Carpenter Technology Corporation
Reading, PA
USA