Higher Performance Material Solutions for a Dynamic Spine Market
White Papers
After cancer and heart disease, back pain is the third most costly medical disorder in the United States. The spinal surgery segment of the orthopedic industry is already the fastest-growing portion of the market, and with changing demographics of the U.S. population, the spinal devices segment is expected to continue to outpace other segments of the industry. Treatment of disorders of the spine has been a very dynamic marketplace for a number of years and is expected to continue over the next decade.
The demographics of the U.S. population are in a state of flux. We are physically larger, more active and living longer than past generations. We want immediate relief of our symptoms and expect spinal implants that not only relieve our pain, but also allow us to continue our active lifestyles. We are receiving implants earlier in life and expect them to perform better and last longer. Clearly, the growing demands on implant performance will require higher performance material solutions.
Given the dynamic nature of the spine segment, in recent years there has been a dramatic increase in the number and types of devices designed for the treatment of the four major types of spinal disorders: deformity disorders, degenerative disorders, spinal trauma and spinal tumors. Each of these disorders presents unique challenges to the surgeon and, likewise, the device designer. However, regardless of the type of disorder being treated, there are two broad-based trends that have been consistent: the need for higher performance/longer lasting implants, and the desire to provide better clinical outcomes for the patient.
The following review of current spinal surgery trends will demonstrate where higher performance alloys and products may be used to satisfy the increased expectations of today’s device designers, surgeons and patients.
Improvements in Fusion/Fixation
Historically, the most frequently selected treatment procedure for many spinal disorders has been spinal fusion and fixation treatments. These treatment options utilize devices such as plates, screws, rods, hooks, spacers and cages. Most of the metals consumed within this sub-segment are titanium-based alloys such as Ti-6Al-4V, Ti-6Al-4V-ELI, Ti-6Al-7Nb, and CP Ti. However, stainless and cobalt are utilized as well.
The components for fusion are typically either machined from round or flat bar stock or cut from flat plate. For that reason, minimizing yield loss during machining and providing starting material closer to near net shape has been an increasing trend. In the case of cervical plates, for example, the titanium flat products offered by Dynamet Incorporated, a subsidiary of Carpenter Technology, have provided consistently closer-to-finish tolerances, improved surface finishes and better shape consistency. There is also an added benefit of enhanced mechanical properties and improved grain structure characteristics when compared to Ti products typically cut from plate (Figure 1).
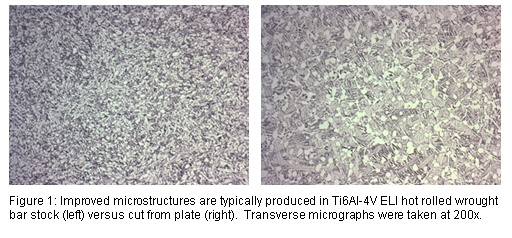
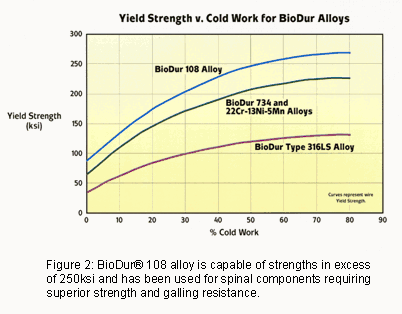
For some fusion applications that utilize stainless rods and screws, medical device designers have requested higher-strength components with enhanced galling resistance. In these cases, BioDur® 108 has proven beneficial. BioDur® 108 alloy is a high-nitrogen, essentially nickel-free stainless that is capable of very high strengths in excess of 200ksi and also demonstrates improved galling resistance when compared to BioDur® 316LS and 22Cr-13Ni-5Mn (Figure 2).
Motion Preservation Technologies
While in many cases traditional fusion/fixation devices have proven successful, they do tend to limit patient mobility. Recently, in order to satisfy one of the needs of the high demand patient, there has been an increasing trend towards non fusion alternatives, including dynamic stabilization, artificial discs, and other motion preserving techniques. In these cases, higher performance alloys may be required that exhibit enhanced wear resistance, finer microstructure or higher fatigue strength. These attributes can be achieved when utilizing alternative alloy processing techniques like Carpenter’s proprietary Micro-Melt® process. In this process, the alloy is melted and then atomized into a fine powder by introducing the molten metal to a high-pressure stream of gas. This powder is then blended and screened to a controlled diameter and finally consolidated into a solid ingot of material which can then be processed to the required finished diameters (Figure 3).
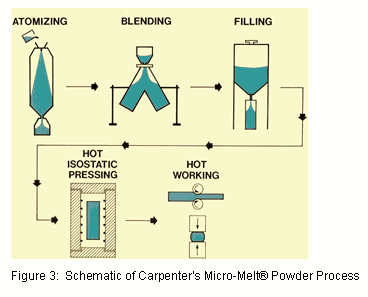
Emerging artificial disc and dynamic stabilization applications have benefited from two high-performance alloys that are manufactured using the Micro-Melt process.
Artificial Discs
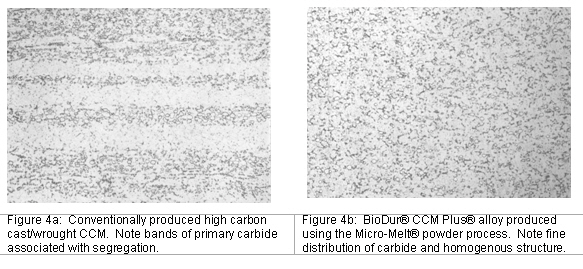
Many disc designs are either already on the market or currently in product development. Some of these designs utilize titanium alloys, some use stainless, some use cobalt, and others use ceramics, PEEK or elastomer components. Each shares one common attribute: the need for a wear-resistant and highly polishable bearing surface. Micro-Melt® BioDur® CCM Plus® has shown to be beneficial for this type of application. Micro-Melt® BioDur® CCM Plus® is manufactured using the Micro-Melt process. As such, it results in an alloy that meets ASTM F1537 Alloy 2 high carbon chemistry, but has the added benefit of superior microstructural uniformity versus other high carbon cobalt-chrome-moly grades on the market (Figure 4a & 4b).
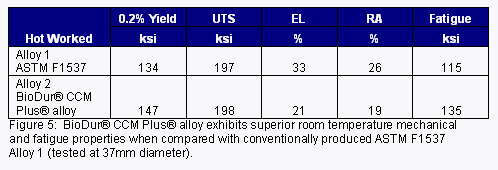
The high-performance microstructure of Micro-Melt® BioDur® CCM Plus® also results in superior fatigue strength and enhanced mechanical properties when compared to the more widely used cast/wrought version of ASTM F1537 Alloy 1 (See Figure 5).
Dynamic Stabilization
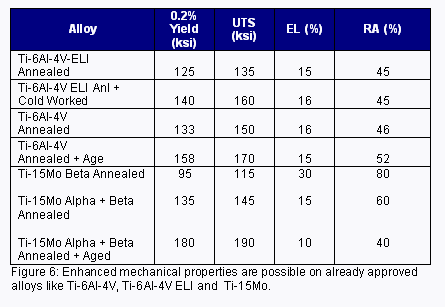
Another active trend in the spine segment is the adoption of dynamic stabilization devices. Like artificial discs, numerous systems are either already introduced, in development or submitted for approval with the FDA. The use of higher performance alloys and/or existing alloys in higher strength conditions has been a trend for these devices. For example, utilizing already approved Ti alloys like Ti-6Al-4V/ELI, Ti-6Al-7Nb and Ti-15Mo in a strain-hardened condition or an annealed + heat-treated condition provides enhanced mechanical properties, which help meet the needs of these more demanding applications (Figure 6).
Deformity Treatment
Another active trend in the spine segment is the adoption of dynamic stabilization devices. Like artificial discs, numerous systems are either already introduced, in development or submitted for approval with the FDA. The use of higher-performance alloys and/or existing alloys in higher-strength conditions has been a trend for these devices. For example, utilizing already approved Ti alloys like Ti-6Al-4V/ELI, Ti-6Al-7Nb and Ti-15Mo in a strain-hardened condition or an annealed + heat-treated condition provides enhanced mechanical properties, which help meet the needs of these more demanding applications (Figure 6).
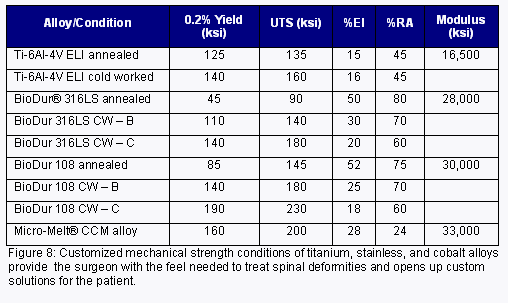
When it comes to the effective treatment of spinal deformities like scoliosis, kyphosis and lordosis, in many cases how an implant feels in the hands of the surgeon becomes a driving factor for the type and condition of the alloy selected for use within the device. The use of higher-performance alloys and alternative strength conditions of already approved alloys has been an increasing trend to satisfy this demand. Specifically, spinal rods for the treatment of spinal deformities have typically utilized stainless steel and titanium; however, cobalt-based alloys are now being used in certain circumstances due to the elevated stiffness, higher modulus and mechanical properties that these alloys possess.
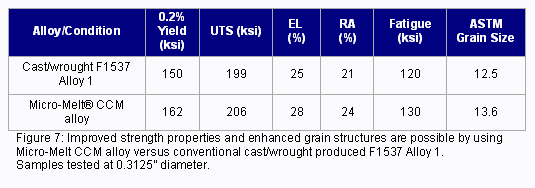
Micro-Melt® BioDur® CCM Plus® has seen increasing use in small-diameter (under 8mm) spinal rod applications. This alloy, manufactured using the proprietary powder process, develops higher strength, finer grain structure and increased fatigue strength over conventional cast/wrought produced ASTM F1537 Alloy 1 cobalt chrome alloys (Figure 7).
In addition to the titanium and cobalt products already mentioned, when designing for deformity treatment the designers should also keep in mind that customized ultra-high strength conditions of current stainless alloys are also available to round out the alloy portfolio (Figure 8). Whether the designer needs titanium, cobalt or stainless as their material solution, options are available that can provide that customized feel that some surgeons require.
Minimally Invasive Surgical Techniques
Another active trend in the spine segment is the adoption of dynamic stabilization devices. Like artificial discs, numerous systems are either already introduced, in development or submitted for approval with the FDA. The use of higher-performance alloys and/or existing alloys in higher-strength conditions has been a trend for these devices. For example, utilizing already approved Ti alloys like Ti-6Al-4V/ELI, Ti-6Al-7Nb and Ti-15Mo in a strain-hardened condition or an annealed + heat-treated condition provides enhanced mechanical properties, which help meet the needs of these more demanding applications (Figure 6).
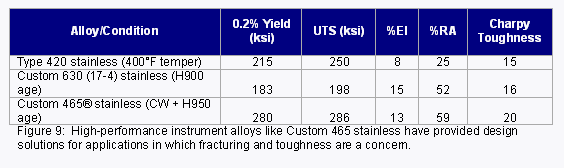
In addition to implants that last longer and provide better solutions for the patient, there has also been a trend toward the adoption of surgical techniques that are less invasive for the patient. Higher performance alloys produced to tighter tolerances can help facilitate MIS technology by allowing for the design of smaller, more durable, more flexible and higher strength instruments and implants. For example, Carpenter’s Custom 465® stainless has helped provide numerous design solutions for instruments that require higher strength and improved toughness (Figure 9). Additionally, Dynamet’s Titanium ULTRABAR product has provided the ultra-tight dimensional tolerances and exceptional diameter uniformity required for some titanium MIS implants. Titanium ULTRABAR can provide diameter tolerance control down to 0.00015” in bar sizes from 3-12mm and lengths up to 14 feet in a variety of implantable titanium alloys.
Summary
Another active trend in the spine segment is the adoption of dynamic stabilization devices. Like artificial discs, numerous systems are either already introduced, in development or submitted for approval with the FDA. The use of higher-performance alloys and/or existing alloys in higher-strength conditions has been a trend for these devices. For example, utilizing already approved Ti alloys like Ti-6Al-4V/ELI, Ti-6Al-7Nb and Ti-15Mo in a strain-hardened condition or an annealed + heat-treated condition provides enhanced mechanical properties, which help meet the needs of these more demanding applications (Figure 6).
Patients who suffer from back pain demand faster recovery times and longer lasting implants. These demands require innovation in both device design and surgical technique. However, innovation does not end there; high performance alloys and products now available in the marketplace and those in current development are helping to satisfy the needs of the device designer, the surgeon and, ultimately, the patient.
Dynamet Incorporated manufactures titanium bar, wire, fine wire and shaped products. Carpenter Technology is a leading manufacturer and distributor of stainless steels, high-strength alloys and other specialty alloys and metallic powders.
***
By: Michael J. Walter
Medical Market Development Manager
Carpenter Technology Corporation, Wyomissing, Pa.