Weaponry
Engineered for orthopedics
Next-generation alloys to advance patient outcomes
Life-changing materials
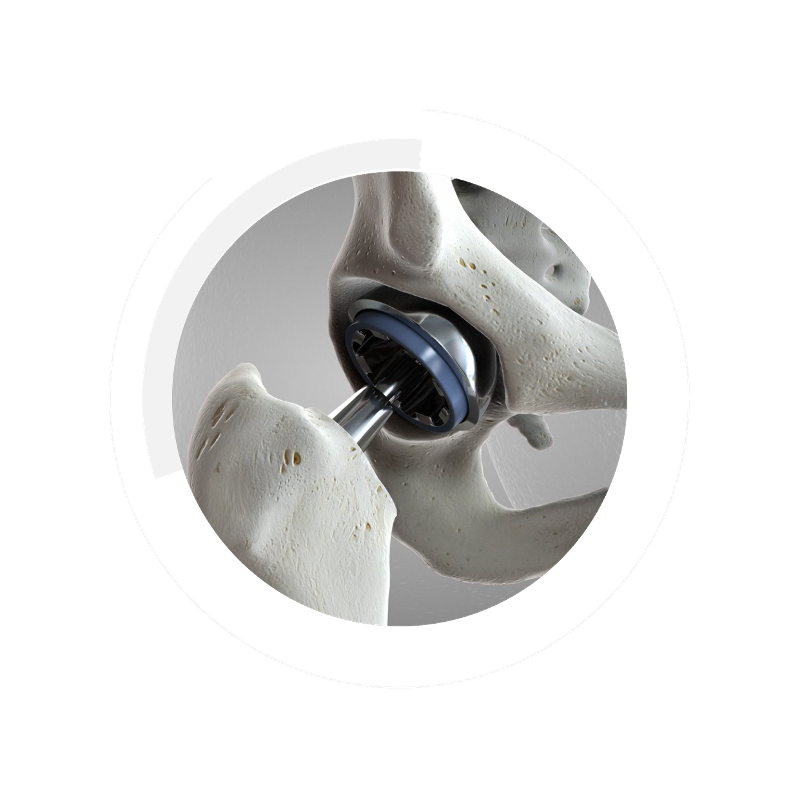
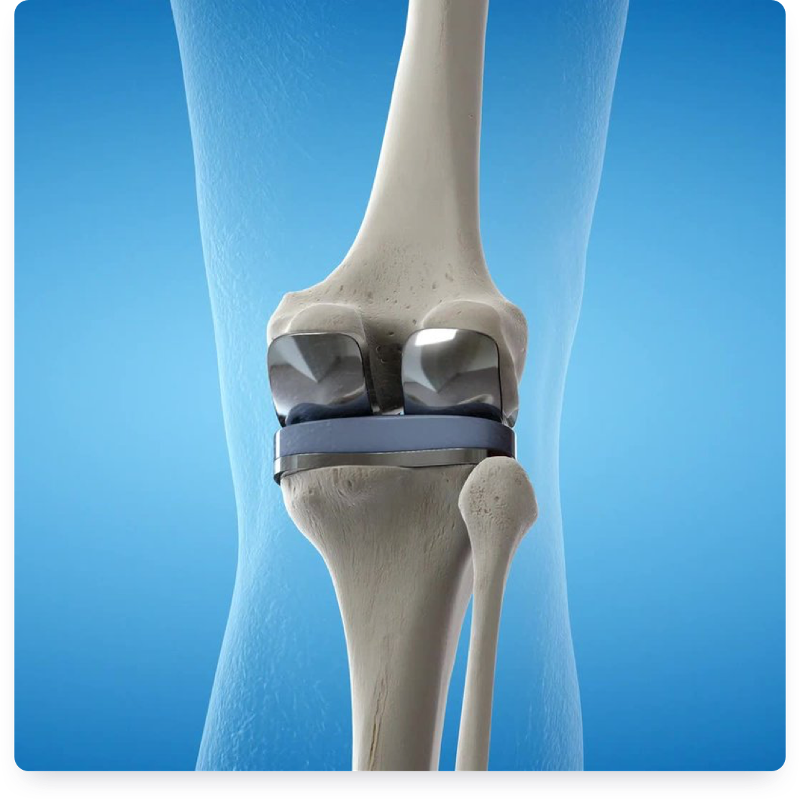
Carpenter Technology produces a range of alloys designed specifically for the rigors of orthopedic devices. Our low-cobalt stainless steels meet EU MDR standards and lead the industry in performance. Our small-diameter materials enable complex manufacturing and less invasive surgery.
NEXT-GEN STAINLESS ALLOYS
High-performance, low-cobalt stainless steel alloys with superior strength and wear resistance meet the demands of new regulatory frameworks, surgical procedures, and production techniques.
- EU MDR-Compliatn to reduce patient exposure
- Built to withstand intense physiological conditions
- Max strength and corrosion resistance with BioDur® 734
ADVANCED CAPABILITIES
High-performance, small-diameter materials meet the tight tolerances required for precision medical devices and enable minimally invasive surgeries and robotic-assisted procedures.
- Tight dimensional tolerances and diameter uniformity
- Unprecedented end-to-end manufacturing consistency
- Cost savings + faster patient recoveries
Next-generation stainless alloys
Stainless. Limitless.
Materials play a prime role in advancing orthopedics to positively impact patient outcomes, reduce procedural cost, meet the demands of new regulatory frameworks, and support an aging population. EU MDR-compliant, low-cobalt stainless steel alloys answer the call of the quickly evolving medical device industry.
Low cobalt for EU MDR compliance
The landscape of the industry is changing with the new European Union medical device regulations (EU MDR). Essentially cobalt-free stainless steel compositions address the up-classification of cobalt as a Class II RMR substance.
Breakthrough BioDur® 734
An EU MDR-compliant, nitrogen-strengthened, austenitic stainless steel, BioDur® 734 is ideal for implantable orthopedic parts with improved tensile, impact, and fatigue strength, and crevice and pitting corrosion resistance over BioDur® 316LS.
Low nickel for patient sensitivities
Exposure to nickel ions released from the normal wear of medical implants can lead to adverse effects for patients sensitive to the metal. BioDur® 108 offers an FDA-approved, essentially nickel- and cobalt-free alternative to other stainless options. Advanced cold-work capabilities allow us to tailor the mechanical properties of BioDur 108® B and C, 316LS, and 465 to specific end uses.
Advanced capabilities
Small diameter. Big impact.
As orthopedic device and surgical tools get smaller and more complex, material choices and manufacturing technologies become ever more crucial. Small-diameter alloys produce industry-leading performance and faster patient recoveries, as well as more efficient, cost-effective manufacturing.
High-performance titanium
With stringent roundness, straightness, and finish requirements, small-diameter titanium delivers tight dimensional tolerances with unprecedented consistency from end-to-end, bar-to-bar, and lot-to-lot.

Intricate cobalt alloy forms
Cobalt-based alloys provide exceptional wear and corrosion resistance, ultra-high tensile strength, and superior ductility and toughness. These properties are vital for creating small-diameter materials and complex forms for minimally invasive procedures.
Powder metallurgy processing
The Micro-Melt powder metallurgy process creates Micro-Melt® BioDur® CCM material with higher strength, enhanced fatigue resistance, increased hardness, improved microstructural uniformity, and finer grain size — allowing for the production of small-diameter stock and custom shapes.

Large-scale nitinol device production
Nitinol is widely used in medical devices due to its superior superelasticity, shape memory effect, low stiffness, damping, biocompatibility, and corrosion resistance. Our Nitinol AM solution empowers large-scale production of complex device geometries with pre-designed porosity, homogeneous composition, high density, and near net shape — all with little to no post-processing.
Driving orthopedic AM
The next great leap in medical device productionis industrializing additive manufacturing (AM). PowderLife is a powder management solutionthat makes AM possible, ensuring quality and reusability, and managing material and inventory.

.jpg)